The Science
Decoding the Complexity of IMIDs
At Invea our vision is clear: to develop safe and effective oral small molecule therapies that target the common pathways of IMIDs. We understand that IMIDs involve a complex cascade of events, from the initial inflammatory triggers to eventual tissue damage and fibrosis. Our approach is driven by the belief that addressing the dysregulated inflammatory response at its core presents a remarkable opportunity for impactful treatments.
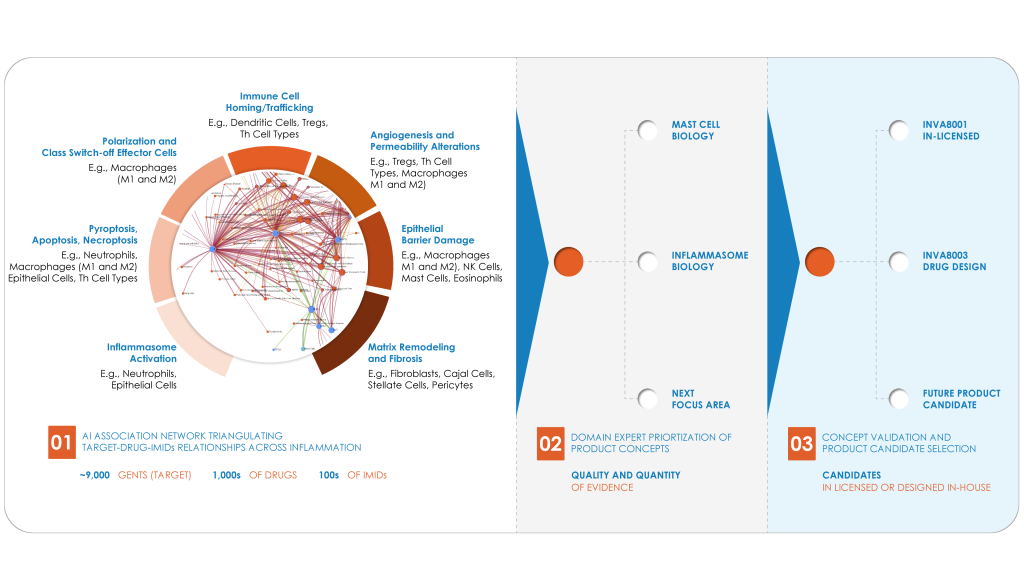
IMIDs are a diverse group of diseases that share common inflammatory pathways and genetic factors. At Invea, we are dedicated to unraveling the intricate science behind these conditions. Our focus areas include:
Mast cells are innate immune cells that play a critical role in chronic inflammation. These cells are widespread throughout the body, including the gastrointestinal tract, liver, and skin, where they help maintain epithelial function and tissue integrity. However, dysregulated mast cell activation can lead to chronic inflammation and the development of IMIDs. We are committed to understanding and targeting mast cell activation to provide effective therapies.
Inflammasomes are intracellular danger-sensing complexes of the innate immune system that assemble in response to infection or tissue injury. Built from upstream sensors (e.g., NLRP family members), the adaptor protein ASC, and pro-caspase-1, inflammasomes drive activation of caspase-1 and release of inflammatory mediators such as IL-1β and IL-18, and can trigger inflammatory cell death. Dysregulated inflammasome signaling has been implicated across multiple IMIDS.
With cutting-edge technology and a passionate team, we aim to leverage the science of IMIDs and pioneer innovative treatments.
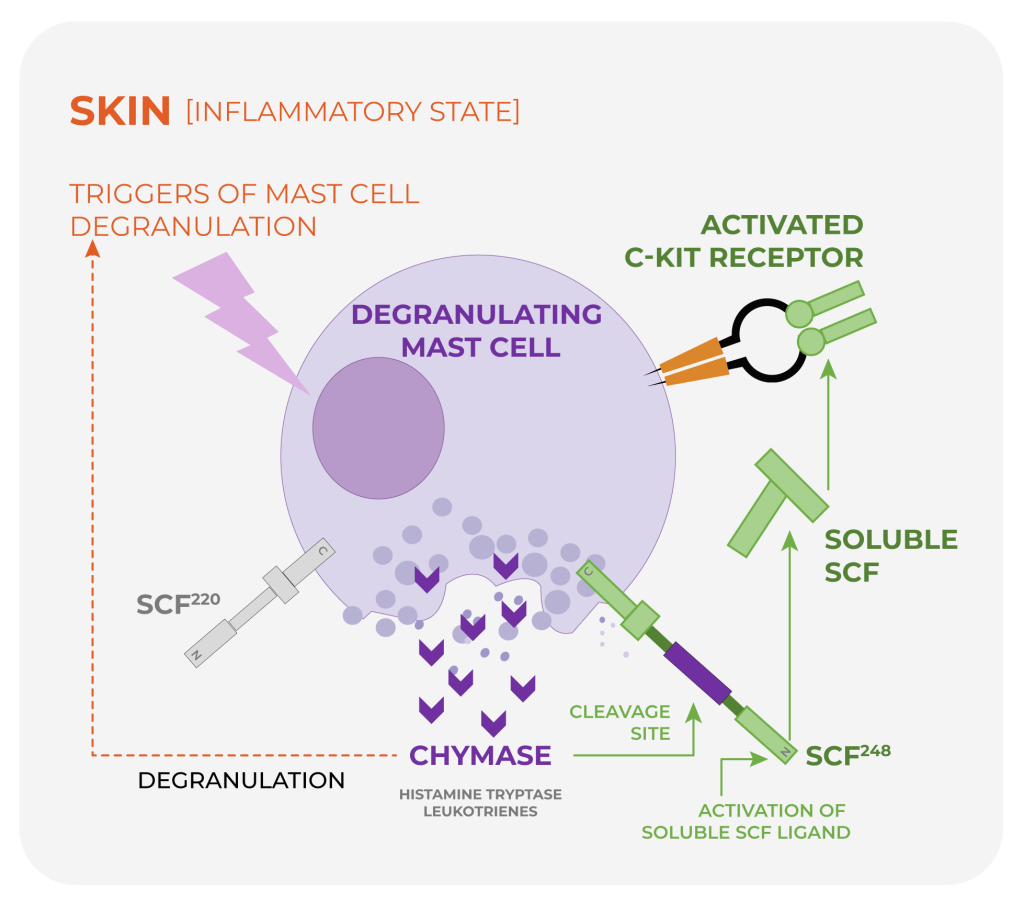
In chronic urticaria and related inflammatory skin and mast-cell disorders, dysregulated mast-cell activation can drive inflammation through degranulation and release of mediators—including chymase, histamine, and tryptase.
SCF–c-KIT signaling is a central pathway for mast-cell activation and proliferation.
- SCF–c-KIT signaling drives mast-cell activation and proliferation.
- Chymase, a mast cell-specific protease, cleaves SCF248 to generate soluble SCF, the ligand that activates c-KIT.
- SCF220 supports normal homeostasis, including hematopoiesis.
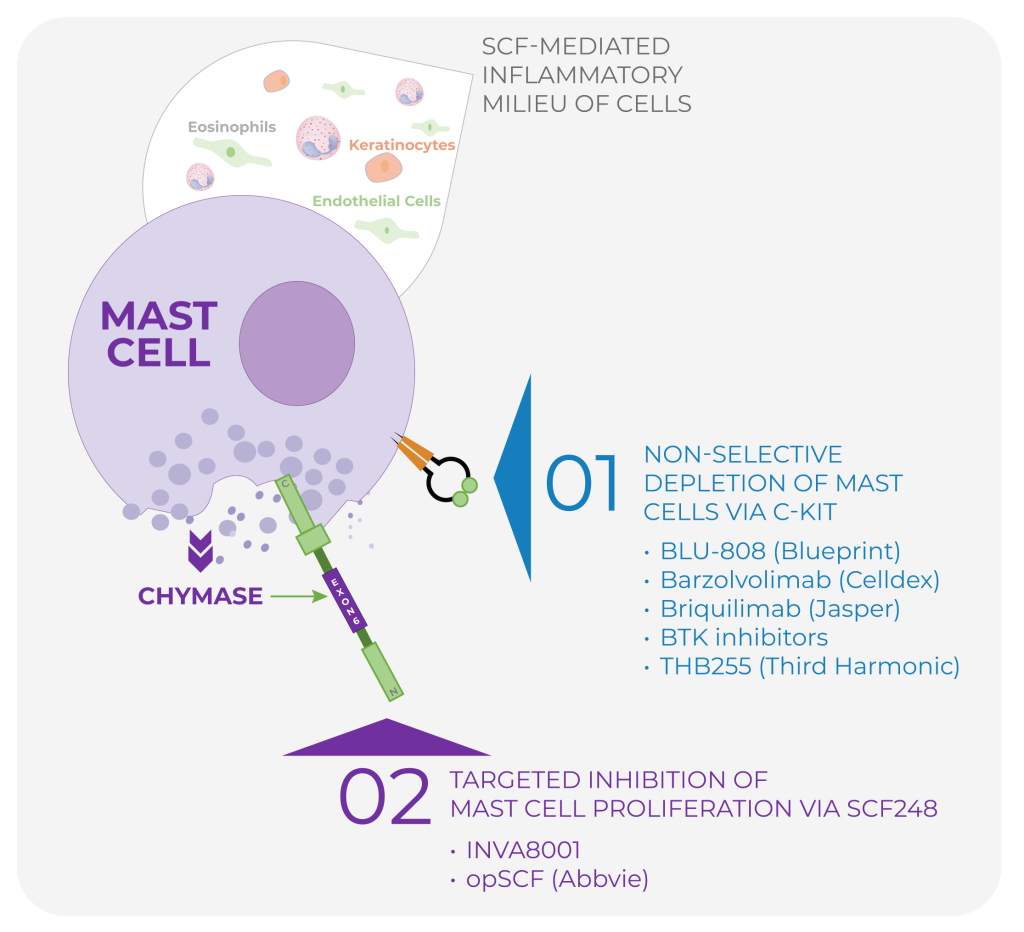
- Current approaches largely rely on c-KIT inhibition to dampen mast-cell activity.
- Because c-KIT is expressed across >10 cell types, blockade can cause systemic liabilities (e.g., neutropenia, hair color changes).
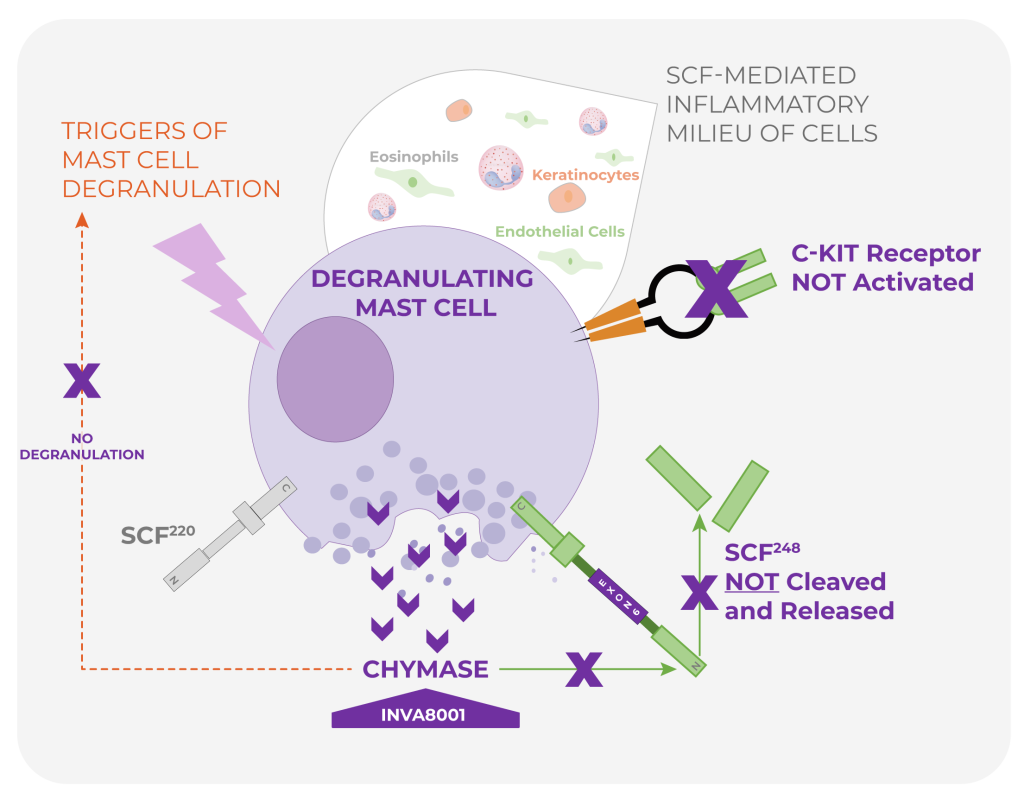
INVA8001 is a selective chymase inhibitor designed to:
- Inhibit SCF248 cleavage, reducing soluble SCF generation.
- Inhibit mast-cell activation, degranulation, and proliferation.
Compared with broad c-KIT inhibition, INVA8001 offers potential advantages including:
- Mast-cell selectivity.
- Selective action on SCF248.
- A novel, potentially safer approach.
- Oral convenience.
- Preservation of key homeostatic signaling, helping avoid systemic liabilities associated with c-KIT blockade.
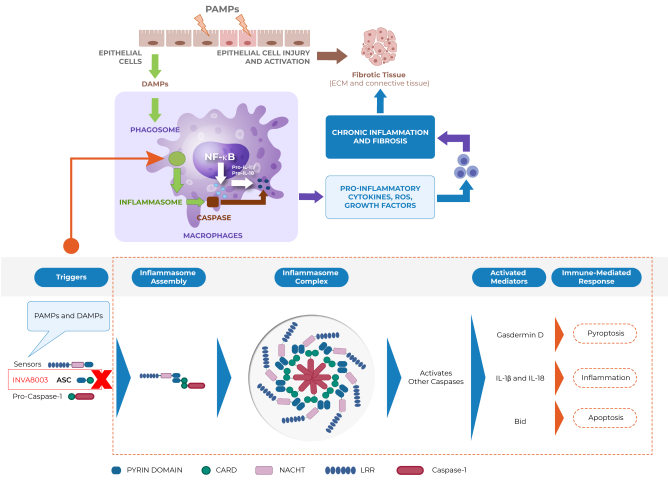
Inflammasome dysregulation has been implicated across multiple IMIDs, where innate immune activation can amplify chronic inflammation via IL-1β/IL-18 release and pyroptosis.
ASC-mediated inflammasome assembly is a central pathway driving this signaling.
- Inflammasomes assemble in immune cells in response to PAMPs/DAMPs.
- They comprise upstream sensors (e.g., NLRP1/3/6/7/12, NLRC4), ASC, and pro-caspase-1.
- ASC links sensors to pro-caspase-1, enabling assembly and caspase-1 activation.
- Activation drives IL-1β/IL-18 maturation/release and can trigger pyroptosis.
- Because ASC is shared across many inflammasomes, targeting ASC may modulate multiple pathways (vs. a single sensor).
INVA8003 is a novel, AI-designed, oral small-molecule multi-inflammasome inhibitor designed to:
- Disrupt ASC-driven protein–protein interactions (including oligomerization) required for inflammasome assembly.
- Inhibit ASC–sensor interactions (e.g., NLRP3), reducing downstream inflammatory signaling.
Compared with single-sensor approaches (e.g., NLRP3-only inhibition), INVA8003 offers potential advantages including:
- Direct disruption of inflammasome assembly via ASC inhibition.
- Multi-inflammasome coverage, potentially supporting a wider therapeutic window than NLRP3-only inhibitors.


